 Methods for reducing intracranial pressure in acute ischaemic stroke | Download Table
Methods for reducing intracranial pressure in acute ischaemic stroke | Download TableWarning: The NCBI website requires JavaScript to operate. Management of intracranial hypertensionAbstract Effective management of intracranial hypertension involves meticulous avoidance of factors that precipitate or aggravate intracranial pressure. When intracranial pressure rises, it is important to rule out new massive injuries that should be surgically evacuated. Medical management of increased intracranial pressure should include sedation, drainage of cerebrospinal fluid and osmotherapy with manitol or hypertonic saline. For intracranial hypertension refractory to initial medical management, barbiturate coma, hypothermia or decompressive craniectomy should be considered. Steroids are not indicated and may be harmful in the treatment of intracranial hypertension resulting from traumatic brain injury. Intracranial hypertension is a common neurological complication in patients with critical disease; it is the common pathway in the presentation of many neurological and non- neurological disorders. The underlying pathophysiology of increased intracranial pressure (ICP) is the subject of intense basic and clinical research, which has led to progress in understanding physiology related to ICP. Few specific treatments for intracranial hypertension have been subjected to randomized trials, however, and most management recommendations are based on clinical experience. Intracranial Pressure Normal values In normal individuals with closed cranial sources, the content of the central nervous system, including brain, spinal cord, blood, brain, and cerebrospinal fluid (CSF), are encased in an uncompatible skull and vertebral channel, forming an almost incompressible system. There is a small amount of training in the system provided by intervertebral spaces. In the average adult, the skull encloses a total volume of 1450 mL: 1300 mL of brain, 65 mL of CSF, and 110 mL of blood []. The Monroe-Kellie hypothesis indicates that the sum of the intracranial volumes of blood, brain, CSF and other components is constant, and that an increase in any of these must be compensated by an equal decrease in another, or if the pressure does not increase. An increased pressure caused by an expanding intracranial volume is distributed evenly throughout the intracranial cavity [,]. The normal range for ICP varies with age. The values for pediatric subjects are not so well established. Normal values are less than 10 to 15 mm Hg for adults and older children, from 3 to 7 mm Hg for young children and from 1.5 to 6 mm Hg for short-term babies. The ICP can be sub-bluster in newborns []. For the purposes of this article, the normal adult PCI is defined as 5 to 15 mm Hg (7.5–20 cm H2O). ICP values from 20 to 30 mm Hg represent mild intracranial hypertension; however, when there is a temporary mass injury, herniation may occur with ICP values less than 20 mm Hg []. ICP values greater than 20 to 25 mm Hg require treatment in most circumstances. The sustained ICP values of more than 40 mm Hg indicate severe intracranial hypertension that threatens life. Overviews of cerebral dynamicsPerfusion blood pressure (CPP) depends on average systemic blood pressure (MAP) and PCI for the following relationship: As a result, PCP can be reduced from an increase in PCI, a decrease in blood pressure or a combination of both factors. Through the normal regulatory process called pressure self-regulation, the brain is able to maintain a normal brain blood flow (CBF) with a CPP of 50 to 150 mm Hg. At CPP values below 50 mm Hg, the brain may not be able to compensate properly, and CBF passively falls with CPP. After injury, the ability of the brain to press self-regulation may be absent or impaired, and even with a normal CPP, CBF may passively follow changes in the CPP. When the CPP is within the normal self-regulatory range (50–150 mmHg), this ability of the brain to press autoregular also affects the ICP response to a change in the CPP [–]. When the pressure self-regulation is intact, the decrease in the results of the CPP in vasodilation of brain vessels, allowing the CBF to remain unchanged. This vasodilation can result in an increase in the PCI, which further perpetuates the decrease in PPP. This response has been called the vasodilatory cascade. Also, an increase in PCP results in the vasoconstriction of the brain vessels and can reduce PCI. When the pressure self-regulation is altered or absent, the PCI decreases and increases with changes in the CPP. Intracranial hypertension Causes of intracranial hypertension Different causes of intracranial hypertension () may occur individually or in several combinations. In the primary causes of PCI growth, PCI normalization depends on rapidly addressing the underlying brain disorder. In the second group, intracranial hypertension is due to an extracranial or systemic process that is often remedial [–]. The last group consists of the causes of PCI increased after a neurosurgical procedure. Box 1. Causes of intracranial hypertension (primary) Brain tumorTrauma (evil and subdural hematoma, contusions of the brain)Normal intracerebral hemorrhageHydrocephaliaDiopatic or benign hypertension intracranial Others (e.g. cerebrious pseudotumor, pneumoencephalus, abscess, cyst) Others (e.g., high-altitude cerebral edema, hepatic insufficiency) Mass injury (hematoma) EdemaIncrease of the volume of cerebral blood (vasodiation) Hypertension disorders CSFIntracranial secondary to traumatic brain injury Special characteristics should be considered in patients with traumatic brain injury (TBI), in which the lesions may be heterogeneous, and several factors often contribute to Recent studies have suggested that cerebral edema is the main cause in most cases []. Often a secondary increase in the ICP is observed from 3 to 10 days after trauma, mainly as a result of late formation of hematomas, such as epidural hematomas, acute subdural hematoma and traumatic hemorrhagic contusions with surrounding edema, sometimes requiring evacuation []. Other potential causes of delayed PCI increases are cerebral vasospasm [], hypoventilation and hyponatremia. Neurological Intensive Care SupervisionIntracranial hypertension is an important cause of secondary lesions in patients with acute neurological and neurosurgical disorders and usually sends specific monitoring. Patients with suspected intracranial hypertension, especially secondary to BIT, should have PCI monitoring; monitoring of the extraction of brain oxygen, such as with ugular bulb oxide or brain tissue PO2, can also be indicated. Patients with brain lesions should also have close control of systemic parameters, including ventilation, oxygenation, electrocardiogram, heart rate, blood pressure, temperature, blood glucose, and fluid intake and output. Patients should be routinely monitored with pulse oxide and capnography to avoid unrecognized hypoxemia and hypoventilation or hyperventilation. A central venous catheter is commonly needed to help evaluate the volume state, and a Foley catheter is used for accurate urine production. Intracranial Pressure MonitoringThe increased clinical symptoms of PCI, such as headache, nausea and vomiting, are impossible to obtain in patients in coma. Papilledema is a reliable sign of intracranial hypertension, but it is rare after head injury, even in patients with documented elevated PCIs. In a study of patients with cranial trauma, 54% of patients had increased the ICP, but only 3.5% had papilloma in the examination of the substanceoscopy []. Other signals, such as pupil-to-warning dilation and the posture of brainbreak, may occur in the absence of intracranial hypertension. Signs of inflammation of the brain, such as the change of middle line and compressed basal cisterns, are predictive of the increase of the ICP, but intracranial hypertension can occur without such findings []. Types of monitors Ventriculostomy catheter is the preferred device to monitor the PCI and the standard against which all newer monitors are compared []. An intraventricular catheter is connected to an external pressure transducer through fluid-filled tubes. The advantages of ventriculostomy are its relatively low cost, the option to use it for the therapeutic drainage of the CSF, and its ability to recalibrate to minimize errors due to the measurement drift. The disadvantages are difficulties with insertion into compressed or displaced ventricles, inaccuracies of pressure measurements due to obstruction of the fluid column, and the need to maintain the transducer at a fixed point of reference relative to the patient's head. The system should be revised for proper operation at least every 2 to 4 hours, and at any time there is a change in the PCI, neurological examination and CSF output. This check should include the assessment of the presence of a proper waveform, which should have respiratory variations and pulse pressure transmitted. When the ventricle cannot be isolated, other alternatives can be used. Non-fluid devices are available for PCI monitoring and have replaced the subarachnoid pernoid. The microsensor transducer and fiber optic transducer are the most widely available. These catheters with transducing tip can be inserted into the subdural space or directly into the brain tissue []. The main advantages of these monitors are the ease of insertion, especially in patients with compressed ventricles; however, none of the catheters with transducing tip can be reset to zero after they are inserted into the skull, and display the measurement drift over time []. Subdural and epidural monitors for PCI measurements are less accurate compared to ventriculostomy monitors or parenchyma. For surgical patients, the ICP monitor may be inserted at the end of the surgical procedure. The surveillance of the ICP remains as long as intracranial hypertension is required, usually 3 to 5 days. A secondary increase in ICP can be observed from 3 to 10 days after trauma in 30% of patients with intracranial hypertension [] secondary to the development of delayed intracerebral hematoma, cerebral vasospasm or systemic factors such as hypoxia and hypotension. Types of intracranial pressure waves The variations observed in the normal tracking of ICP originate from small pulses transmitted from systemic blood pressure to intracranial cavity. These blood pressure pulses overlap with the slower oscillation caused by the respiratory cycle. In mechanically ventilated patients, the pressure in the upper vena cava increases during inspiration, which reduces the venous flow of the skull, causing an elevation in the PCI. Pathological waveforms As ICP increases, brain compliance decreases, blood pulses become more pronounced and venous components disappear. Pathological waveforms include Lundberg A, B and C. Lundberg Waves or plateau waves are ICP elevations to more than 50 mm Hg from 5 to 20 minutes. These waves are accompanied by a simultaneous increase in MAP, but it is not clear whether the change in MAP is cause or effect. Lundberg B waves or pressure pulses have a range of 50 mm Hg and occur every 30 seconds to 2 minutes. Lundberg C waves have a width of 20 mm Hg and a frequency of 4 to 8 per minute; they are seen in the normal ICP waveform, but high-density C waves can be overlapped in plateau waves []. PCI monitoring is an invasive technique and has some associated risks. For a favorable risk-benefit ratio, PCI monitoring is indicated only in patients with significant risk of intracranial hypertension [] (). IBT patients who are particularly at risk of developing a high ICP include those with a Glasgow Coma Scale of 8 or less after cardiopulmonary resuscitation and who have an abnormal head CT scan. Such anomalies could include low density or high density lesions, including bruises; epidural, subdural or intraparenquimal hematomas; compression of basal cisterns; and edema []. Patients who are able to follow commands have a low risk of developing intracranial hypertension, and neurological examinations can be followed in series. Box 2: Indications for the ICP MonitoringGCS Score: 3-8 (after resuscitation) TC of the abnormal admission head ScanHematomaContusionEdemaHerniation Compressed basal cells Normal Admission Head of TC scanner PLUS 2 or more of the followingAge √≥ 40 years Engine posture Exact blood pressure Although the results of the non-intramousal blood pressure developer. 60% of patients with closed head injury and an abnormal tomography have intracranial hypertension. Only 13% of patients with normal computed tomography have elevated the PCI except for patients with certain risk factors, including the age above 40 years, systolic blood pressure below 90 mm Hg, and decerebrate or decorticate the position in the motor test. Patients with normal computed tomography have 60% risk of intracranial hypertension if they have two risk factors and 4% if they only have a risk factor. Patients with a Glasgow Coma Scale score of more than 8 could also be considered for PCI monitoring if they require treatment that would not allow serial neurological examinations, such as prolonged anesthesia for multiple lesions or extended pharmacological paralysis for ventilation management, or if they require treatment that could increase PCI, such as positive final pressure (PEEP). Other less common indications include patients with multiple systemic lesions with altered level of consciousness and after the elimination of an intracranial mass (e.g., hematoma, tumor) []. PCI surveillance should also be considered in non-traumatic conditions in which an intracranial mass injury (e.g., cerebral infarction, spontaneous intracerebral bleeding) is presented and has a probability of expansion leading to intracranial hypertension and clinical deterioration. The duration of monitoring is until the ICP has been normal for 24 to 48 hours without ICP therapy. Complications of intracranial pressure monitoring The most common complication of the placement of ventriculostomy catheter is the infection with an incidence of 5% to 14%; the colonization of the device is more common than the clinical infection []. A study did not find a significant reduction in the rate of infection in patients who experienced prophylactic changes before day 5, compared to those whose catheters were in force for 5 days or more.[] Factors that are not associated with the infection are the insertion of the catheter into the neurological ICU, the prior insertion of the catheter, the drainage of the CSF and the use of steroids. In a group of patients with prolonged ventricular drainage of 10 days or more, a non-linear increase in the daily infection rate was observed during the first 4 days, but it remained constant despite the prolonged use of the catheter []. It has been shown that the use of antibiotic ventriculostomy catheters reduces the risk of infection from 9.4 to 1.3 per cent []. Other complications of ventricultomy catheters are bleeding with a general incidence of 1.4%, malfunction, obstruction and malposition. Intracranial pressure treatment measures: short summary of the goals of therapyKeep PCI less than 20 to 25 mm Hg.Keep PCC to more than 60 mm Hg while maintaining an appropriate MAP. Avoid factors that aggravate or precipitate the elevated ICP. A general approach to the management of intracranial hypertension is presented in the Diagram of Diagnosis and Treatment of Intracranial Hypertension. General attention to minimizing intracranial hypertensionPrevention or treatment of factors that can aggravate or precipitate intracranial hypertension is a cornerstone of neurological critical attention. Specific factors that can aggravate intracranial hypertension include obstruction of venous return (head position, agitation), respiratory problems ( respiratory tract obstruction, hypoxia, hypercapnia), fever, severe hypertension, hyponatremia, anemia and seizures. Optimization of cerebral vein output To minimize resistance to venous output and promote CSF displacement from the intracranial compartment to the spinal compartment, lifting the head of the bed and keeping the head in a neutral position are standard in neurosurgical care. Some authors have advocated keeping the patient's flat head to maximize the CPP []. Other studies have shown a reduction in ICP without a reduction in PPP or CF in most patients with head elevation to 30° []. Other authors have noted that the elevation of the head to 30° reduced the PCI and increased the PPP, but did not change the oxygenation of the brain tissue []. The reduction in the ICP offered by 15° to 30° head elevation is probably advantageous and safe for most patients. When the head lift is used, pressure transducers for blood pressure and PCI should be zero at the same level (at the Monro foramen level) to evaluate the CPP accurately. Increased intraabdominal pressure, as may occur with abdominal compartment syndrome, may also exacerbate PCI presumably by obstructing brain venous flow. Several case reports have observed immediate reductions in ICP with decompressive laparotomy in such circumstances. A retrospective report indicated that even when abdominal compartment syndrome is not present, abdominal facial release can effectively reduce PCI that is refractory to medical treatment []. Respiratory insufficiency Respiratory dysfunction is common in patients with intracranial hypertension, especially when the cause is cranial trauma. Hypoxia and hypercapnia can dramatically increase PCI and mechanical ventilation can alter brain hemodynamics. Optimal respiratory management is crucial for ICP control. Thirty-six percent of patients with head injury in a coma present hypoxia and respiratory dysfunction that require mechanical ventilation in admission. Pneumonia and pulmonary insufficiency occur in 42% and 28% as complications during hospitalization. In 227 patients with spontaneously breathing neurological disorders, most with intracranial hypertension, North and Jennett found that 60% had respiratory abnormalities, including periodic breathing, tachypnea and irregular breathing []. However, periodic breathing was not correlated with any particular anatomical site of neurological injury. Periodic episodes of hypoventilation may precipitate the increase of PCI []. The controlled ventilation to maintain a normal PaCO2 can eliminate this cause of intracranial hypertension. Mechanical ventilation may also have adverse effects on ICP. PEEP, which may be necessary to improve oxygenation, can increase PCI by preventing venous return and increasing cerebral venous pressure and PCI, and decreasing blood pressure, resulting in increased reflex of brain blood volume. In order for PEEP to increase brain venous pressure to levels that would increase PCI, cerebral venous pressure should at least match PCI. The higher the PCI, the higher the PEEP will have a direct hydraulic effect on the PCI. The consequences of PEEP in the ICP also depend on lung compliance, and the minimum consequences for ICP are usually observed when lung compliance is low as in patients with acute lung injury [].Sedation and analgesiaEasing and pain can significantly increase blood pressure and ICP. Appropriate sedation and analgesia is an important attached treatment. No sedative regime has clear advantages in this patient population. In general, benzodiazepines cause a coupled reduction in the cerebral metabolic oxygen (CMRO2) and CBF rate, with no effect on the ICP, while narcotics have no effect on CMRO2 or CBF, but it has been reported that they increase ICP in some patients []. A consideration in the choice of sedative should be to minimize the effects on blood pressure because most available agents can lower blood pressure. Hypovolemia predisposes to hypotensive side effects and should be treated before administering sedative agents. The selection of shorter acting agents may have the advantage of allowing a brief interruption of sedation to evaluate the neurological state. FeverFever increases the metabolic rate from 10% to 13% by Celsius grade and is a powerful vasodilator. Fever-induced dilation of the brain vessels can increase the FBC and can increase the PCI. The fever during the post-injury period worsens the neurological lesion in the experimental models of BIT []. In an observational study in patients with BIT, Jones and colleagues [] found a significant relationship between fever and a poor neurological result. While a patient is at risk of intracranial hypertension, the fever should be controlled with antipyrtics and cooling blankets. Infectious causes should be sought and treated with appropriate antibiotics when present. Hypertension High blood pressure is commonly observed in patients with intracranial hypertension, especially secondary to head injury, and is characterized by increased systolic blood pressure greater than diastolic increase. It is associated with sympathetic hyperactivity []. It is unwise to reduce systemic blood pressure in patients with hypertension associated with untreated intracranial mass lesions because brain perfusion is maintained by higher blood pressure. In the absence of an intracranial mass injury, the decision to treat systemic hypertension is more controversial and it may be necessary to individualize for each patient. When self-regulation of the pressure is affected, which is common after BIT, systemic hypertension can increase BBC and ICP. In addition, high blood pressure can exacerbate brain edema and increase the risk of postoperative intracranial bleeding. Systemic hypertension can be solved with sedation. If the decision is made to treat systemic hypertension, the choice of antihypertensive agent is important. Vulodylating drugs, such as nitroprusside, nitroglycerin and nifedipin, can be expected to increase PCI and can reflexively increase plasma catecholamines, which can be harmful to the brain that is marginalized. Antihypertensive drugs of sympathetic blockage are preferred, such as β blocking drugs (labetalol, smolol) or central action α-receptors (clonidine), because they reduce blood pressure without affecting PCI. Agents with a short average life have an advantage when blood pressure is lip. Anemia TreatmentAnecdotal cases of patients with severe anemia have been reported with symptoms of PCI increase and signs of papiloma, which resolve with anemia treatment []. It is believed that the mechanism is related to the marked increase in CCI that is required to maintain the delivery of brain oxygen when the anemia is severe. Although anemia has not been clearly shown to exacerbate ICP after IBD, a common practice is to maintain the concentration of hemoglobin at a minimum of 10 g/dL. In view of a large randomized trial of patients with critical disease that showed better result with a more restrictive transfusion threshold of 7 g/dL [], the problem of optimal concentration of hemoglobin in patients with IBT needs further study. Prevention of seizures The risk of seizures after trauma relates to the severity of the brain injury; seizures occur in 15% to 20% of patients with severe cranial injury. Seizures may increase the metabolic rate of brain and PCI, but there is no clear relationship between the occurrence of early seizures and a worse neurological result []. In patients with severe BIT, 50% of seizures can be subclinical and can only be detected with continuous electroencephalographic monitoring []. Significant risk factors for subsequent seizures are contusions, subdural hematoma, depressed cranial fracture, penetrating head wound, loss of consciousness or amnesia for more than 1 day and 65 years or more. In a randomized clinical trial, phenitoin reduced seizures during the first week after trauma, but not later []. Based on this study, seizure prophylaxis is recommended for patients with severe brain injury during the first 7 days after injury. Treatment with anticonvulsants beyond 7 days should be reserved for patients who develop late seizures [].Measures for refractory intracranial hypertension For patients with sustained ICP elevations of more than 20 to 25 mm Hg, additional measures are needed to control ICP. Emerging surgical management should be considered when intracranial hypertension suddenly occurs or is refractory to medical management. Medical Interventions Heavy Sedation and ParalysisPernatal paralysis of patients with neurosurgical disorders is not indicated; however, intracranial hypertension caused by agitation, posture or cough can be prevented by sedation and non-polarizing muscle relaxants that do not alter cerebrovascular resistance []. A commonly used regimen is morphine and lorazepam for analgesia/sedation and cisatracurium or vecuronium as a muscle relaxant, with the dose titted by twitch response to stimulation. Although a disadvantage of this therapy is that neurological examination cannot be monitored closely, sedatives and muscle relaxers can be interrupted once a day, usually before morning rounds, to allow neurological evaluations. The main complications of neuromuscular blocking are myopathy, polyneuropathy and prolonged neuromuscular blockage. Myopathy is associated with the use of neuromuscular block agents, especially in combination with corticosteroids []. Polyneuropathy has been observed in patients with sepsis and multiple organ failure. Prolonged neuromuscular blocking is observed in patients with multiple organ failure, especially with kidney and liver dysfunction. The recommendations to minimize these complications are limiting the use and dosage of neuromuscular block agents, monitoring four trains, measurement of phosphokinase creatine daily, and stopping the drug daily to evaluate the motor response []. Hyperosmolar TherapyMannitol is the most used hyperosmolar agent for the treatment of intracranial hypertension. More recently, hypertonic saline has also been used in this circumstance. Some studies have compared the relative effectiveness of these two hyperosmotic agents, but more work is needed. Intravenous manitol bolt management lowers PCI in 1 to 5 minutes with a peak effect at 20 to 60 minutes. The effect of the manitol on the PCI lasts 1.5 to 6 hours, depending on the clinical condition []. Manitol is usually given as a 0.25 g/kg to 1 g/kg bw; when an urgent reduction of ICP is required, an initial dose of 1 g/kg bw should be given. Hypotension (systolic blood pressure Mannitol has retological and osmetic effects. Immediately after the infusion of manitol, there is an expansion of the plasma volume and a reduction in hematocrit and the viscosity of blood, which can increase the FBC and in balance increase the delivery of oxygen to the brain. These retological effects of manitol depend on the state of self-regulation pressure []. In patients with intact pressure self-regulation, the infusion of manitol induces cerebral vasoconstriction, which keeps CBF constant, and the decrease of PCI is large. In patients with absent pressure self-regulation, manitol infusion increases the FBC, and the decrease in PCI is less pronounced. Mannitol can also improve microcirculatory reology [] and has free radical scam effects. The osmotic effect of the manitol increases the tonicity of the serum, which extracts edema fluid from the cerebral parenchyma. This process takes 15 to 30 minutes until gradients are established. The osmolarity of the serum seems to be optimal when it increases to 300 to 320 mOsm and should remain less than 320 mOsm to avoid side effects of therapy, such as hypovolemia, hyperosmolarity and kidney failure. Mannitol opens the blood-brain barrier, and the manitol that has crossed the blood-brain barrier can draw fluid to the central nervous system, which can aggravate vasogenic edema. For this reason, when it is time to stop the manitol, it should be recorded to avoid a rebound in brain edema and PCI. The adverse effects of manitol are very likely when manitol is present in circulation for long periods, such as in slow or continuous infusions or with repeated doses administration higher than necessary. Hypertonic saline, given in concentrations ranging from 3% to 23.4%, also creates an osmotic force to extract water from the interstitial space of the cerebral parenchyma in the intravascular compartment in the presence of an intact blood barrier, reducing intracranial volume and PCI. In some studies, hypertonic saline has been more effective in reducing PCI than manitol [,]. Hypertonic saline has a clear advantage over manitol in hypovolemic and hypotensive patients. Manitol is relatively contraindicated in hypovolemic patients due to diuretic effects, while the hypertonic increase of saline increases the intravascular volume and may increase blood pressure in addition to lowering PCI. Hypertonic saline was not associated with better neurological results, however, when it was given as a prehospital bolt to hypotensive patients with severe BIT. The adverse effects of the hypertonic saline administration include hematological and electrolytic abnormalities, such as secondary bleeding to decreased plaquetal aggregation and prolonged coagulation, hypokalemia and hyperchloromal acidosis []. Hypoatremia should be excluded before administering hypertonic saline to reduce the risk of central myelinolysis of pontin.[] HyperventilationPaCO2 decreases hyperventilation, which may induce constriction of brain arteries by alkalinizing CSF. The resulting reduction in the volume of brain blood decreases the ICP. Hyperventilation has a limited use in the management of intracranial hypertension, however, because this effect on the ICP is limited by time, and because hyperventilation can lead to a sufficient decrease in the CF to induce ischemia. The vasoconstrictive effect on brain arteries lasts only 11 to 20 hours because the CSF pH quickly balances the new PaCO2 level. As the CSF pH balances, the cerebral arteries are redirected, possibly to a greater caliber than at the base, and the initial reduction in the volume of brain blood comes at the expense of a possible phase of rebounding the PCI [,]. For this reason, the most effective use of hyperventilation is acutely to allow other more definitive treatments to be implemented. When hypocarbia is induced and maintained for several hours, it should be slowly reversed for several days to minimize this rebounded hyperemia []. Hyperventilation decreases the CF, but if this reduction in flow is sufficient to induce ischemia in the injured brain is controversial. A prospective study showed that sharply reducing the PaCO2 from an average of 36 mm Hg to 29 mm Hg reduced the global CBF and significantly increased the volume of the brain that was markedly hypoperfused despite improvements in ICP and CPP []. The syringe and the coworkers [] showed similar changes in the FBC with moderate hyperventilation, but did not notice changes in the regional CMRO2, even when the FQC was reduced to less than 10 mL/100 g/min in brain tissue, suggesting that the energy failure associated with cerebral ischemia was not happening. Although hyperventilation induced ischemia has not been clearly demonstrated, routine chronic hyperventilation (to PaCO2 of 20–25 mm Hg) had a harmful effect on the result in a randomized clinical trial []. The authors of this study recommended the use of hyperventilation only in patients with intracranial hypertension, rather than as a routine in all patients with head injuries. This vision is reinforced in the BIT guidelines. In barbiturate comaClustered coma should only be considered for patients with refractory intracranial hypertension due to severe complications associated with high-dose barbiturates, and because the neurological examination becomes indisposable for several days []. Pentobarbital is given in a load dose of 10 mg/kg bw followed by 5 mg/kg bw per hour per 3 doses. The maintenance dose is 1 to 2 mg/kg/h, tetred at a serum level of 30 to 50 μg/mL or until the electroencephalogram shows a pattern of pharmase suppression. Although the routine use of barbiturates in unselected patients has not been consistently effective in reducing morbidity or mortality after severe head injuries [,], a randomized multicenter trial showed that the establishment of barbiturate coma in patients with refractory intracranial hypertension resulted in a double greater possibility of controlling ICP []. The ICP reduction mechanism by barbiturates is not clear, but it probably reflects a reduction coupled within the framework of CBF and CMRO2, with an immediate effect on ICP. Studies of Messeter and colleagues [,] have suggested that the reduction of PCI with barbiturates is closely linked to the retention of the reactivity of carbon dioxide by the brain. Complications that occur during treatment with barbiturate coma include hypotension in 58% of patients, hypokalemia in 82%, respiratory complications in 76%, infections in 55%, liver dysfunction in 87%, and renal dysfunction in 47% []. The hypotension caused by pentobarbital should be treated first with volume replacement and then with vasopressors if necessary. Experimental studies suggest that for the treatment of hypotension associated with barbiturate coma, volume revival may be better than dopamine [] because dopamine infusion increased brain metabolic requirements and partially offsets the beneficial effects of barbiturates in CMRO2. Hypotemia Although a multicentered, moderate hypothermia randomized clinical trial in severe IB did not show a beneficial effect on the neurological outcome, it was observed that fewer randomized patients with moderate hypothermia had intracranial hypertension []. A randomized pilot hypothermia trial in children with BIT produced similar results—no improvement in the neurological outcome, but a reduction in ICP during the treatment of hypothermia []. Although the routine induction of hypothermia is not currently indicated, hypothermia may be an effective adjunctive treatment to increase the refractory of ICP to another medical management. SteroidsSysteroids are commonly used for primary brain tumors and metastases, to decrease vasogenic brain edema. Focal neurological signs and decreased mental state due to surrounding edema usually begin to improve within hours []. The increase in ICP, when present, decreases in the next 2 to 5 days, in some cases to normality. The most commonly used regime is intravenous dexamethasone, 4 mg every 6 hours. For other neurosurgical disorders, such as BIT or spontaneous intracerebral hemorrhage, steroids have not shown a benefit [,] and in some studies have had a harmful effect [,]. The CRASH [] trial is a randomized, placebo-controlled trial of methylprednisolone for 48 hours in patients with BIT recently performed, ample (with 10,000 registered patients). Methylprednisolone administration resulted in a significant increase in the risk of death from 22.3 per cent to 25.7 per cent ( relative risk 1.15, confidence interval of 95% 1.07–1.24). This trial confirmed earlier studies and guidelines that routine steroid administration is not indicated for patients with BIT. Surgical interventionsResection of mass lesions The intracranial masses that produce the elevated PCI must be eliminated whenever possible. Acute epidural hematomas and subdurals are a hyperagute surgical emergency, especially epidural hematoma because the bleeding is under blood pressure. Brain abscess should be drained, and pneumococphaly should be evacuated if it is under enough tension to increase PCI. Surgical management of intracerebral bleeding is controversial []. cerebrospinal fluid drainage CSF drainage immediately decreases PCI by reducing intracranial and long-term volume allowing edema fluid to drain into the ventricular system. The drainage of even a small volume of CSF can significantly reduce PCI, especially when intracranial compliance is reduced by injury. This modality can be an important adjuvant therapy to reduce ICP. If the brain is swollen diffusely, the ventricles can collapse, and this modality then has a limited utility. decompressive craniectomy The surgical removal of part of the calvary to create a window in the cranial vault is the most radical intervention for intracranial hypertension, denying the Monro-Kellie doctrine of the fixed intracranial volume and allowing the herniation of the brain. Decompressive craniectomy has been used to treat uncontrolled intracranial hypertension of various origins, including brain infarction [], trauma, subarachnoid bleeding and spontaneous bleeding. Selection of patients, time of operation, type of surgery and severity of clinical and radiological brain injury are all factors that determine the result of this procedure. Sahuquillo and Arikan [] reviewed the evidence in literature for studies that evaluate the effectiveness of decompressive craniectomy after BIT. They found only a small randomized clinical trial in 27 children with BIT []. This trial found a reduced risk ratio for the death of 0.54 (confidence interval of 95% 0.17–1.72), and a risk ratio of 0.54 for death, vegetative state or severe disability of 6 to 12 months after injury (95% confidence 0.29–07). All studies available in adults are case series or cohorts with historical controls. These reports suggest that decompressive craniectomy effectively reduces PCI in most patients (85%) with intracranial hypertension refractory to conventional medical treatment [,]. Cerebral oxygenation measured by PO2 tissue and blood flow estimated by the rate of average brain flow is also improved after decompressive craniectomy [,]. Reported complications include hydrocephalus, ipsilateral hemorrhagic inflammation to the site of craniectomy, and subdural higroma []. A paradoxical hernia case has also been reported after a lumbar puncture in a patient with a decompressive craniectomy []. There are limited results of randomized trials to confirm or refute the effectiveness of decompressive craniectomy in adults. Reports suggest, however, that decompressive craniectomy may be a useful option when maximal medical treatment has failed to control ICP. There are randomized controlled trials in BIT (Rescue ICP [] and DECRAN). In a joint analysis of randomized trials in patients with malignant MCA infarction, decompressive surgery performed at 48 h of stroke was associated with the reduction of mortality and a higher proportion of patients with a favorable functional result []. ResumenThe effective treatment of intracranial hypertension involves meticulous avoidance of factors that precipitate or aggravate the increase of the ICP. When the PCI rises, it is important to rule out new massive injuries that should be surgically evacuated. The increased medical management of PCI should include sedation, drainage of CSF and osmotherapy with manitol or hypertonic saline. For intracranial hypertension refractory to initial medical management, barbiturate coma, hypothermia or decompressive craniectomy should be considered. Steroids are not indicated and may be harmful in the treatment of intracranial hypertension resulting from BIT. This article was supported by NIH Grant P01-NS38660. Footnotes This PDF receipt will only be used as a basis for generating PubMed Central (PMC) documents. PMC documents will be made available for review after conversion (approximately 2-3 weeks). Any corrections required will be made at that time. No material shall be released to PMC without the approval of an author. Only PMC documents will appear in PubMed Central -- this PDF receipt will not appear in PubMed Central. Disclaimer from the editor: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will be submitted to copy, composition and revision of the resulting test before it is published in its final citable form. Please note that during the production process you can discover errors that could affect the content, and all legal claims that apply to the journal. ReferencesFormats: Share , 8600 Rockville Pike, Bethesda MD, 20894 USA
Intracranial pressure increase: What to KnowThe growing intracranial pressure is a medical term that refers to the growing pressure within a person's skull. This pressure can affect the brain if doctors don't treat it. A sudden increase in pressure within a person's skull is a medical emergency. Without treatment, an increase in intracranial pressure (ICP) can cause brain injury, seizures, , , or death. With quick treatment, it is possible that people with the greatest PCI will make a complete recovery. In this article we examine the symptoms, causes and treatments of the increased PCI. Symptoms of ICP increase may vary depending on the age of a person. Babies with older PCI may have symptoms different from older children or adults with the condition, as described below. Symptoms in adults Symptoms of PCI enhancement in adults include: As the elevated PCI progresses, a person may lose consciousness and enter a coma. High PCI can cause brain damage if a person does not receive emergency treatment. Symptoms in Babies Older PCI babies can show some of the same symptoms as adults. In addition, the shape of your heads can be affected. Babies still have soft plates in their skull that fibrous tissue called skull sutures are weaved together. The increase of the PCI can make the skull sutures split and the soft plates move. PCI's increase in babies can also cause your fontanel to bulge. The fontanel is the soft spot on the top of the skull. Below is a list of medical conditions and other causes that can lead to a larger ICP: In infants, the high ICP may be the result of child abuse. If a person handles a baby or a baby too hard, it can cause them to develop a brain injury. This is known as shaken baby syndrome. One source has estimated that between the United States they experience shaken baby syndrome every year. The condition may arise if an adult violently shakes a baby to stop them crying. Anyone who suspects that a child may be experiencing abuse may contact anonymity at 1-800-4-A-CHILD (1-800-422-4453). If a person has the symptoms of increased PCI, you should see a doctor immediately. This is a medical emergency and can cause brain injury if a person does not receive rapid treatment. A doctor will measure ICP in millimeters of mercury (mm/Hg). The normal range is . When the PCI passes over this, a person may be experiencing an increase in the PCI. To diagnose the ICP increase, a doctor may ask if a person has: Then, the doctor may perform the following tests: After these initial tests, the doctor may use one to examine the brain tissue of a person in more detail. If a person has an increased ICP diagnosis, a doctor will work immediately to reduce pressure inside the skull to lower the risk of brain damage. Then they will work to treat the underlying cause of increased pressure. Treatment methods to reduce PCI include: A doctor may give the person a sedative to help reduce and reduce his/her . The person may also need breathing support. Your doctor will monitor your vital signs throughout your treatment. In rare cases, the doctor may put a person with a high PCI in a coma induced by doctors to treat his condition. Increased PCI complications include:Without proper treatment, increased PCI may be fatal. A sudden increase in ICP is a medical emergency and can be life-threatening. The sooner a person gets treatment, the better their perspective will be. Many people respond well to treatment, and a person who has experienced a major PCI can make a full recovery. The increase in ICP is not always preventable, but it is possible to reduce the risk of some underlying conditions that can lead to a larger ICP. We explored how down. StrokeStroke can cause PCI increase. A person can reduce his or her risk of stroke in the following ways: High blood pressure High blood pressure can cause PCI rise. A person can maintain healthy blood pressure by: A head injury can cause PCI boost. Examples of how a person can reduce his or her risk of head injury are: PCI increases when the pressure within a person's skull increases. When this happens suddenly, it's a medical emergency. The most common cause of high ICP is a blow to the head. The main symptoms are headache, confusion, decreased alertness and nausea. A person's students cannot respond to the light in the usual way. An older PCI person may need urgent treatment. The immediate goal of treatment is to reduce pressure on your brain tissue, which helps reduce the risk of brain damage. Without proper treatment, this condition may cause seizure, coma, stroke or brain damage. In serious cases, the increase in ICP may be fatal. Rapid treatment can improve a person's perspectives. Making a complete recovery with timely treatment is possible. The increase in ICP is not always preventable, but a person can reduce their risk of some causes by changing lifestyle. Last medical review on January 11, 2019Most recent newsRelated coverage

Reducing intracranial pressure in patients with traumatic brain injury - American Nurse
Increased Intracranial Pressure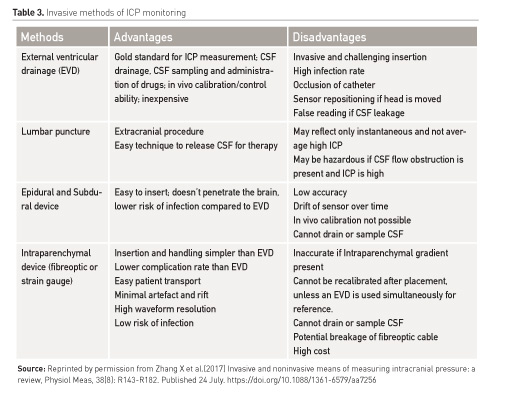
Intracranial pressure monitoring devices - HealthManagement.org
Reducing intracranial pressure in patients with traumatic brain injury - American Nurse
Management of patient with increased intracranial pressure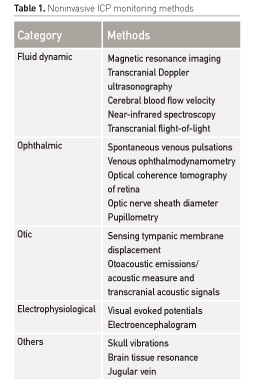
Intracranial pressure monitoring devices - HealthManagement.org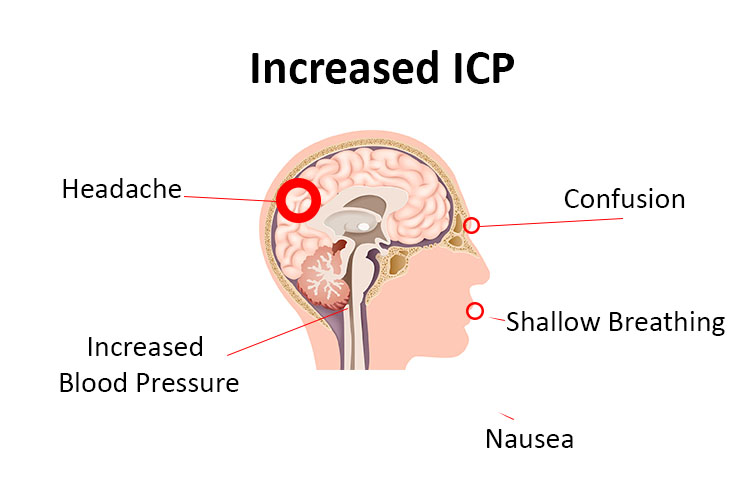
Increased Intracranial Pressure: A Guide For Nurses | Ausmed
Management of patient with increased intracranial pressure
New therapies for intracranial hypertension
Osmotherapy - Wikipedia
Potential strategies for management of intracranial pressure and... | Download Scientific Diagram
Management of patient with increased intracranial pressure
New therapies for intracranial hypertension
New therapies for intracranial hypertension
Traumatic Intracranial Hypertension | NEJM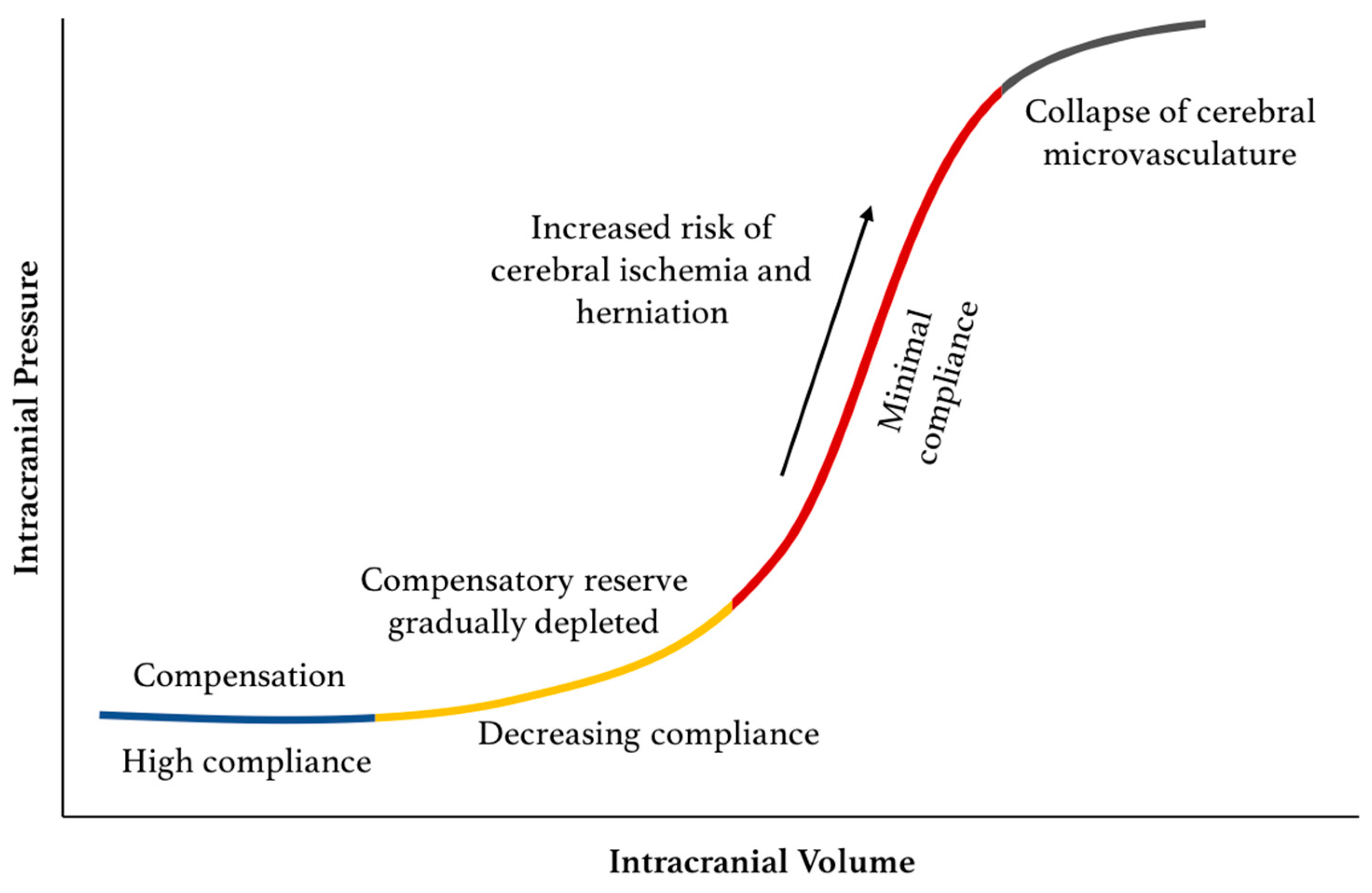
Sensors | Free Full-Text | Intracranial Pressure Monitoring—Review and Avenues for Development | HTML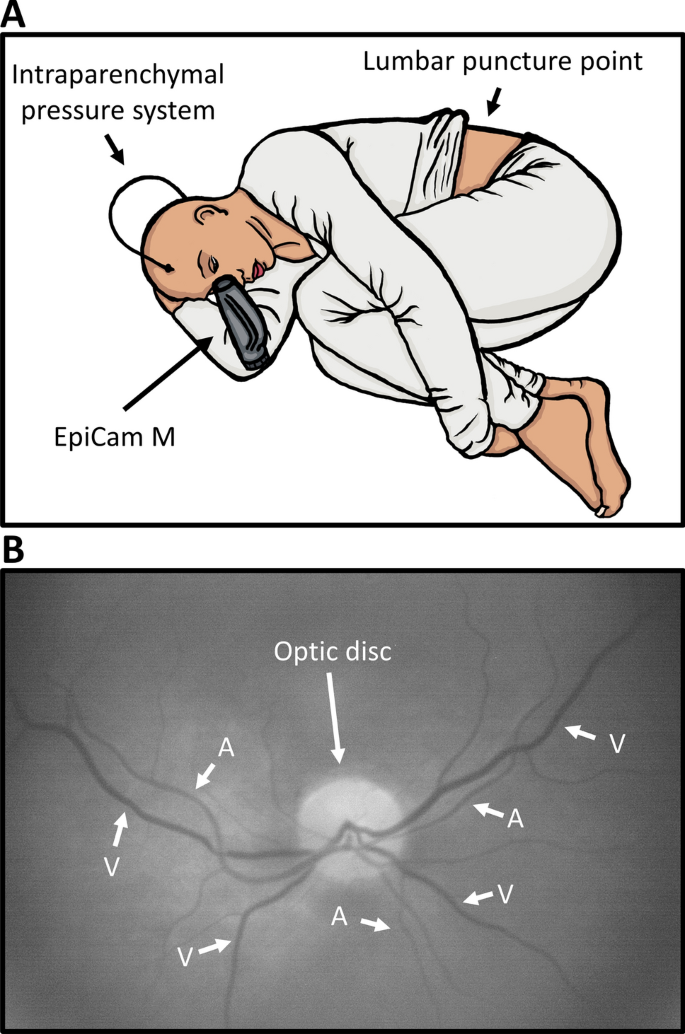
A new novel method for assessing intracranial pressure using non-invasive fundus images: a pilot study | Scientific Reports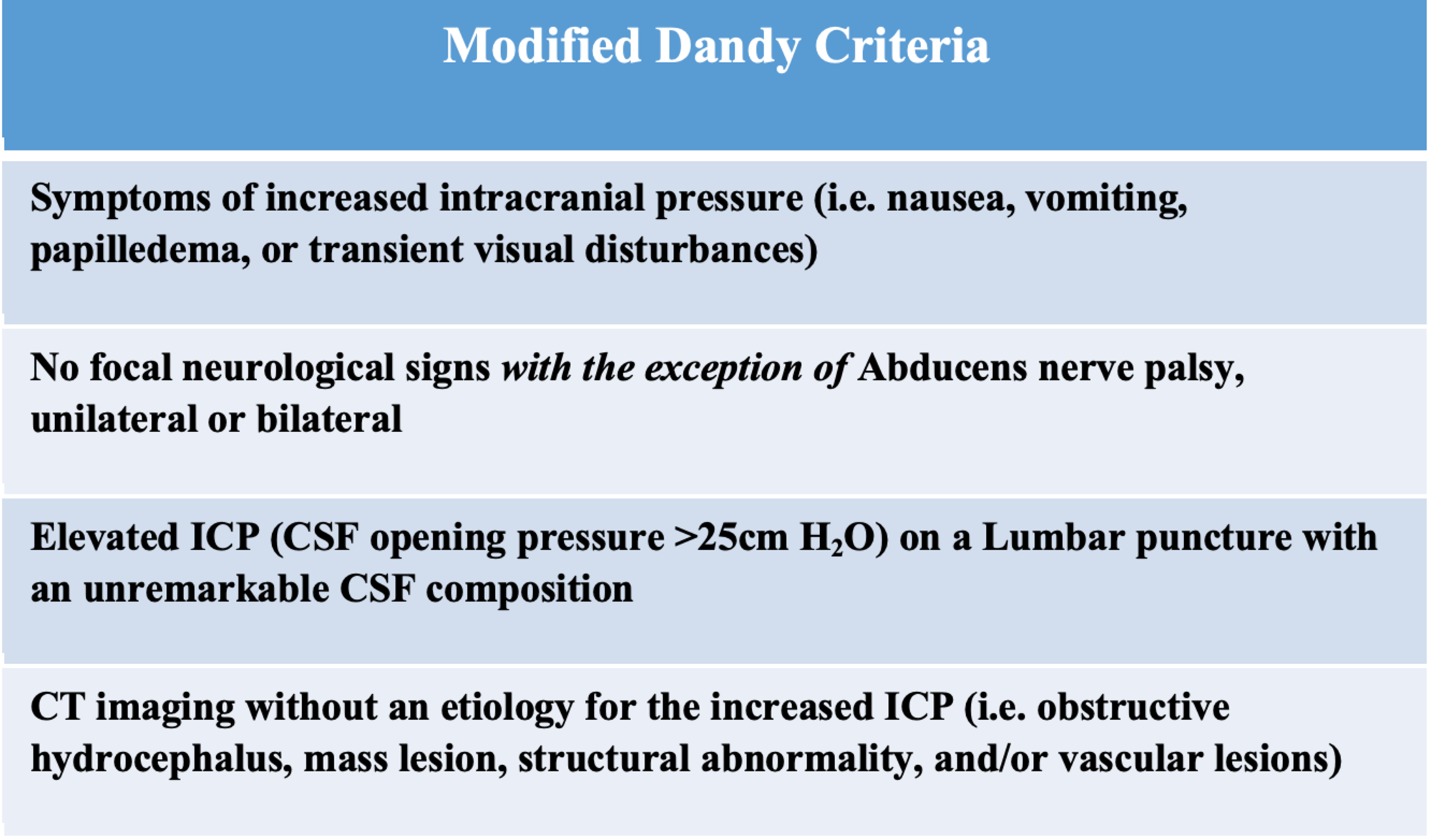
Cureus | Intracranial Venous Sinus Stenting: A Review of Idiopathic Intracranial Hypertension and Expanding Indications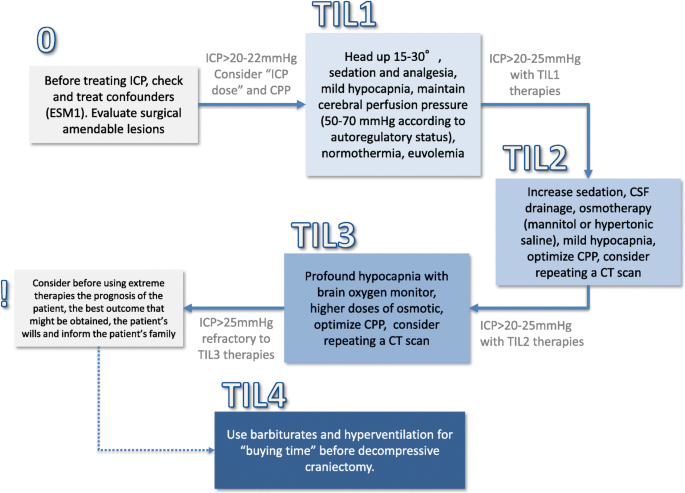
How I manage intracranial hypertension | Critical Care | Full Text
Stepladder approach to the management of intracranial hyper tension... | Download Scientific Diagram
Reducing intracranial pressure in patients with traumatic brain injury - American Nurse
Raised intracranial pressure and seizures in the neurological intensive care unit - ScienceDirect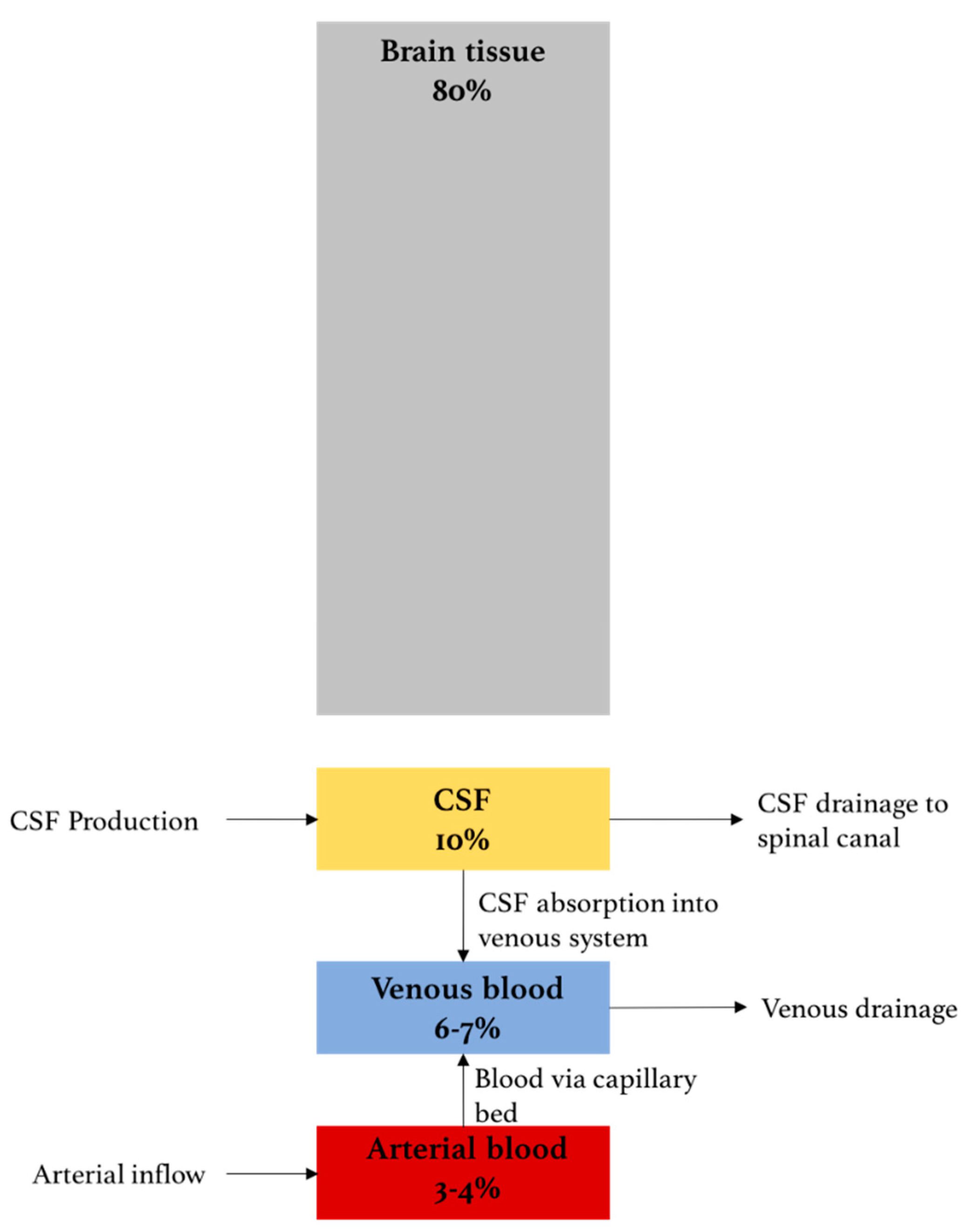
Sensors | Free Full-Text | Intracranial Pressure Monitoring—Review and Avenues for Development | HTML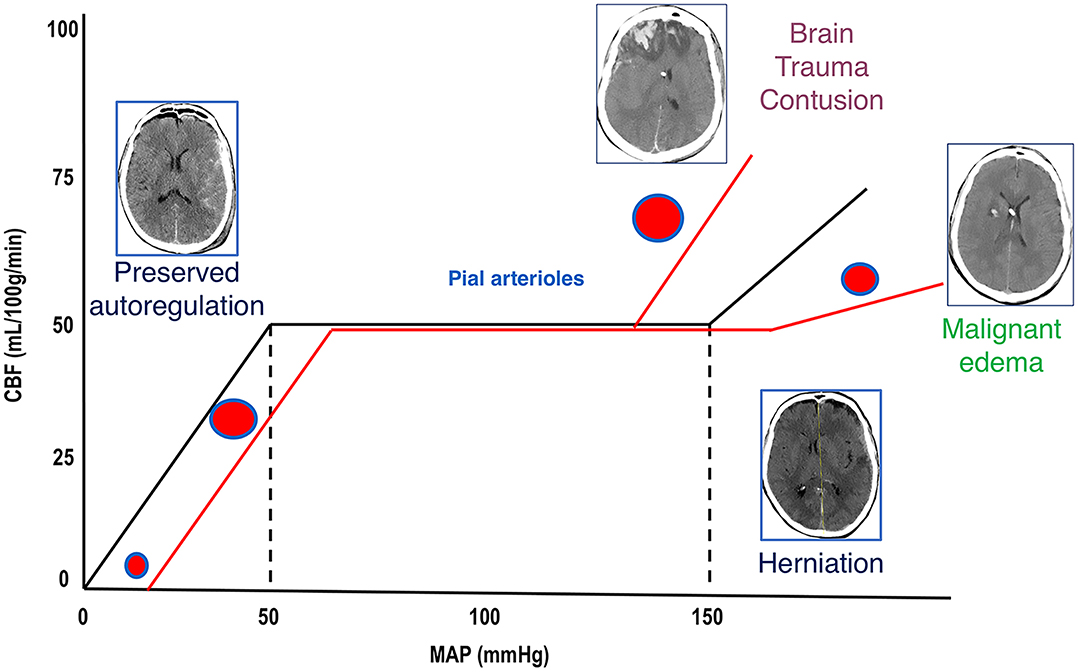
Frontiers | Escalate and De-Escalate Therapies for Intracranial Pressure Control in Traumatic Brain Injury | Neurology
Idiopathic intracranial hypertension: consensus guidelines on management. - Abstract - Europe PMC
Traumatic Intracranial Hypertension | NEJM
PDF) Approach to an increased intracranial pressure
Management of raised intracranial pressure in children with traumatic brain injury. - Abstract - Europe PMC
Evolving evidence in adult idiopathic intracranial hypertension: pathophysiology and management | Journal of Neurology, Neurosurgery & Psychiatry
Diagnosis of Elevated Intracranial Pressure in Critically Ill Patients - EMOttawa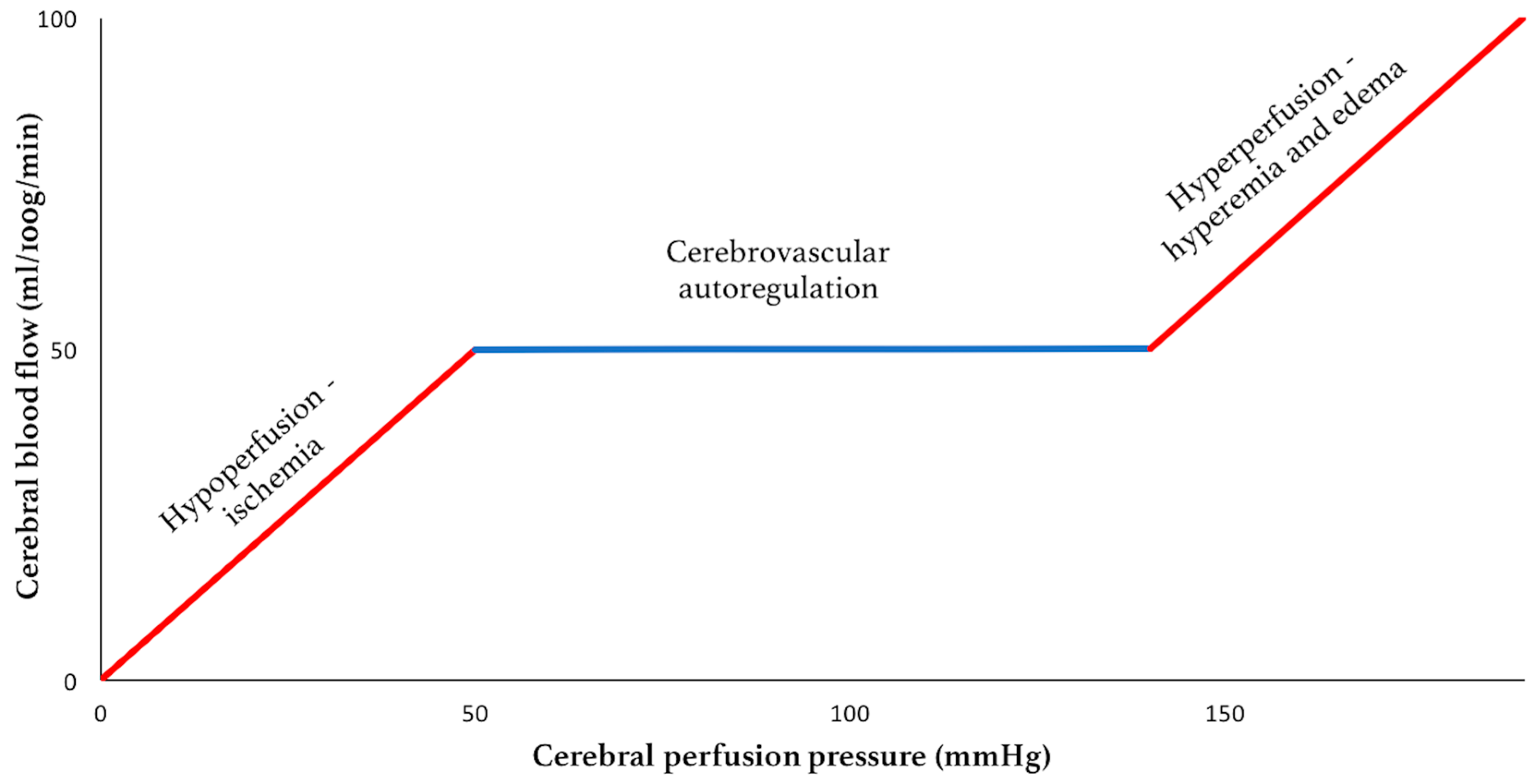
Sensors | Free Full-Text | Intracranial Pressure Monitoring—Review and Avenues for Development | HTML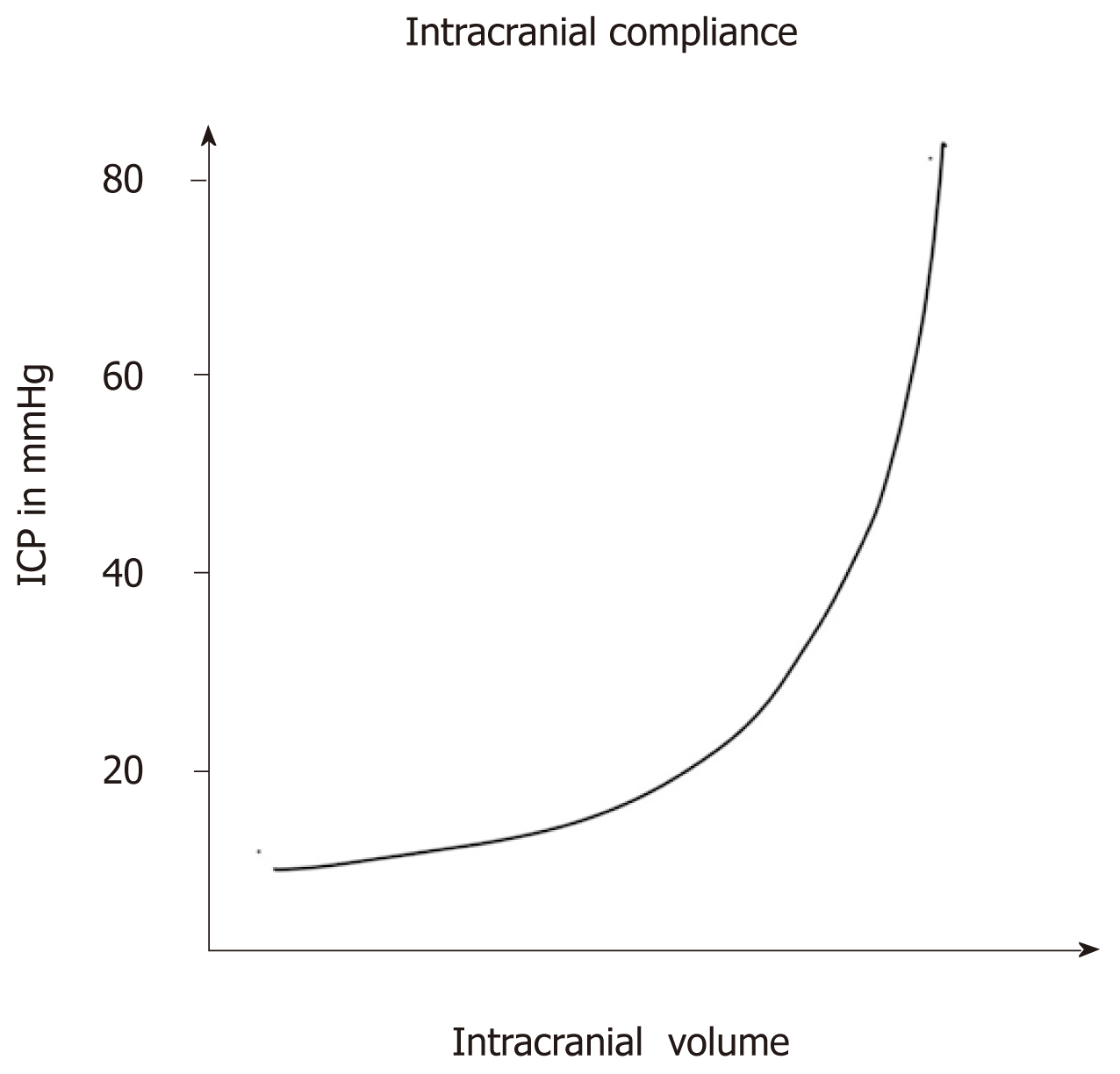
Intracranial pressure monitoring: Gold standard and recent innovations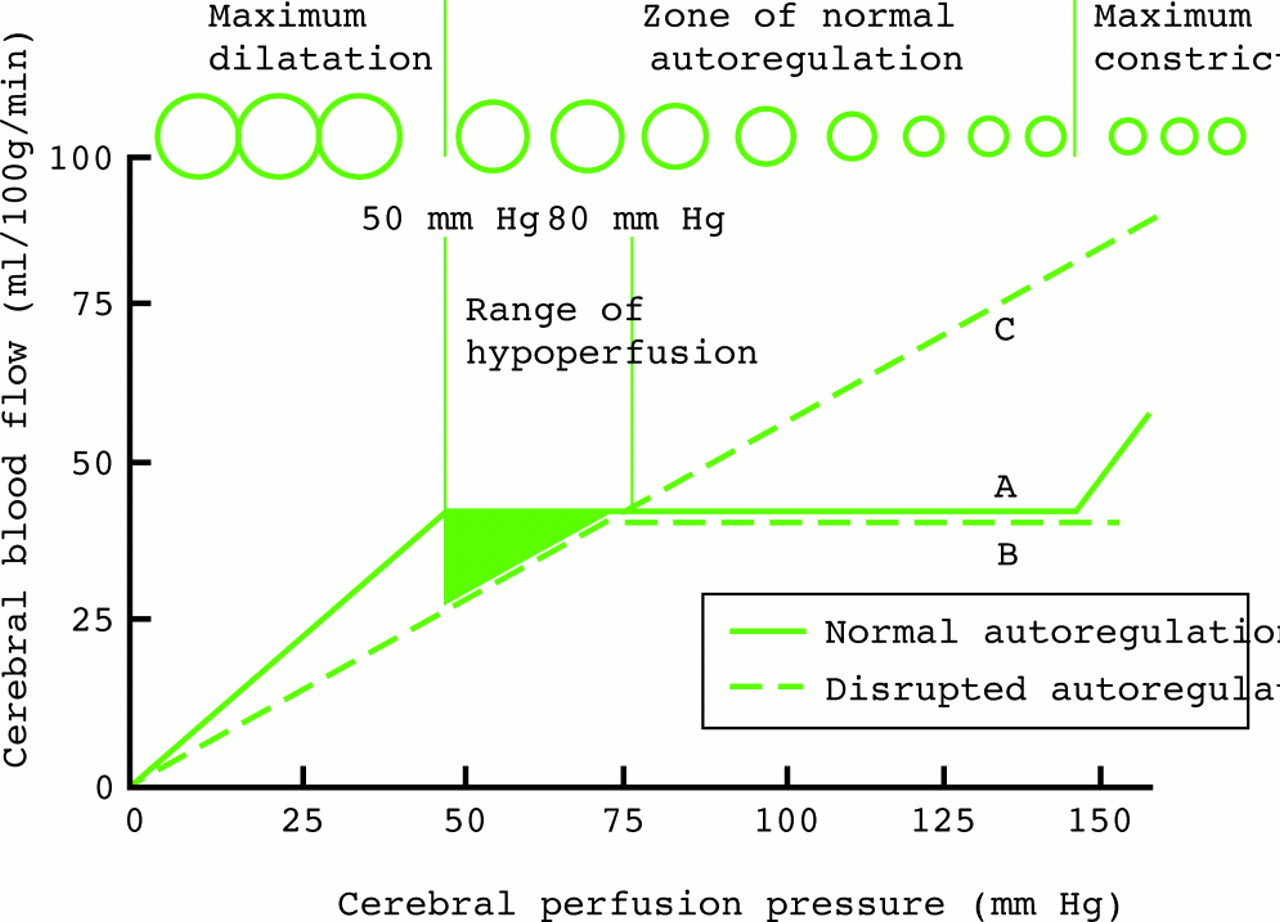
RAISED INTRACRANIAL PRESSURE | Journal of Neurology, Neurosurgery & Psychiatry
Idiopathic Intracranial Hypertension - Mayo Clinic Proceedings
Oral Glycerol for the Reduction of Intracranial Pressure in: Journal of Neurosurgery Volume 21 Issue 4 (1964)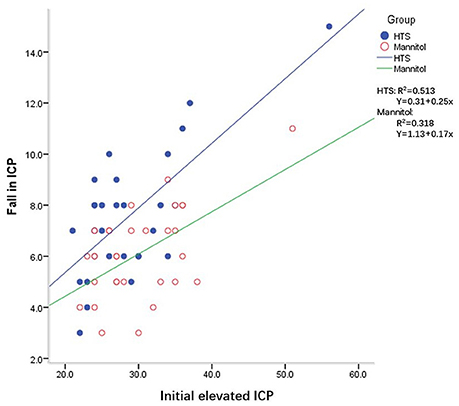
Frontiers | A Retrospective Study of Intracranial Pressure in Head-Injured Patients Undergoing Decompressive Craniectomy: A Comparison of Hypertonic Saline and Mannitol | Neurology
Raised intracranial pressure: What it is and how to recognise it | Roytowski | Continuing Medical Education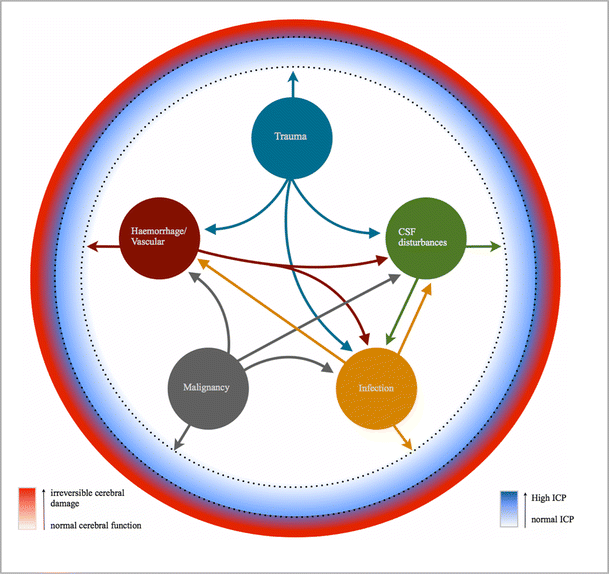
The best marker for guiding the clinical management of patients with raised intracranial pressure—the RAP index or the mean pulse amplitude? | SpringerLink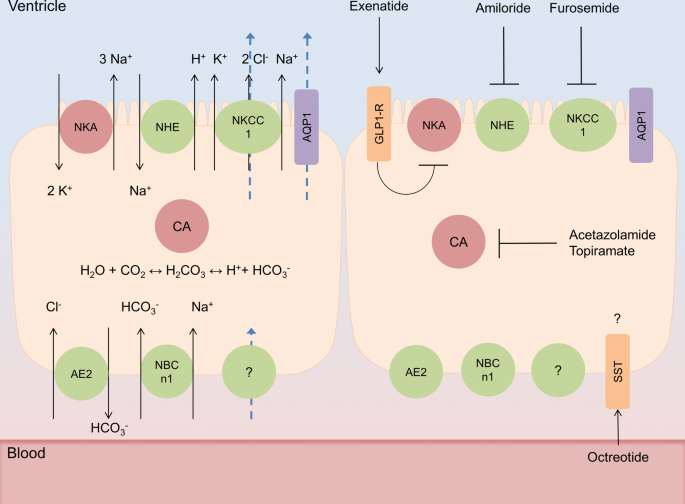
Preclinical update on regulation of intracranial pressure in relation to idiopathic intracranial hypertension | Fluids and Barriers of the CNS | Full Text
The role of ICP monitoring in meningitis in: Neurosurgical Focus Volume 43 Issue 5 (2017)
 Methods for reducing intracranial pressure in acute ischaemic stroke | Download Table
Methods for reducing intracranial pressure in acute ischaemic stroke | Download Table


































Posting Komentar untuk "which method is used to help reduce intracranial pressure?"